Abstract
INTRODUCTION:
Chemotherapy-induced thrombocytopenia (CIT) is a common complication of cancer therapy. Due to the lack of FDA-approved therapies for CIT and clear limitations of platelet transfusions, clinicians often must choose between reducing dose intensity, delaying treatment, switching to an alternative regimen less prone to thrombocytopenia, or even discontinuing therapy altogether. Lower overall survival in CIT is associated with bleeding complications in patients proceeding with chemotherapy as well as patients managed with reductions in relative dose intensity of chemotherapy due to dose reduction and/or treatment delay. Although there are no current FDA-approved therapies for CIT, evidence has emerged supporting use of romiplostim to treat CIT. However, predicting clinical response to romiplostim before initiation is not currently possible due to the lack of a known predictive biomarker. Baseline endogenous TPO levels may, however, be a predictive biomarker for response to thrombopoietin receptor agonists. Prior studies have demonstrated the utility of baseline TPO level to predict romiplostim response in ITP and avatrombopag response in thrombocytopenia of chronic liver disease. Because measurement of TPO level prior to romiplostim treatment of CIT is standard at our institution, we investigated the utility of baseline TPO level to predict romiplostim response in CIT.
METHODS:
We performed an observational study of patients age ≥18 years who had TPO levels measured and received romiplostim for the treatment of CIT. For each weekly on-romiplostim platelet (Plt) count assessment, clinical response was defined as Plt ≥75×10 9/L and ≥30×10 9/L above pretreatment baseline. Overall, moderate, and superior classes of treatment response were defined based on response fraction (RF) of weekly Plt assessments meeting clinical response criteria (RF >0, ≥ 0.6, and ≥ 0.8, respectively). Median TPO levels were compared in those patients with and without a moderate response or a superior response using the Mann-Whitney U test. A generalized linear model was used to model the logit transformation of RF to predict response fraction based on TPO level. Receiver operating characteristic (ROC) analysis was performed to identify TPO level thresholds for optimal discrimination of those who achieved moderate and superior responses from those who did not.
RESULTS:
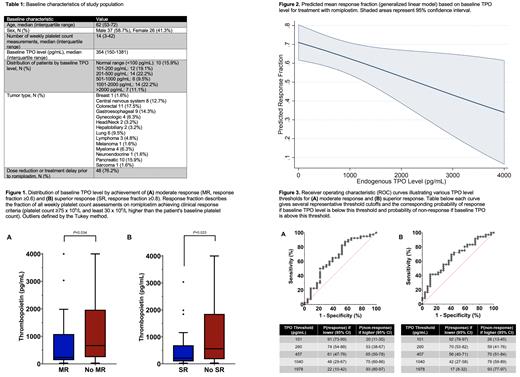
Patients: 63 patients met inclusion criteria. Baseline patient characteristics are detailed in TABLE 1.
Relation of TPO Level and Romiplostim Response: Median (IQR) TPO level was lower in patients who achieved a moderate response to romiplostim versus those who did not, 234 (135-1085) pg/mL versus 665 (244-1970) pg/mL (P=0.034) and lower still in patients who achieved a superior response versus those who did not, 212 (91-690) pg/mL versus 559 (173-1851) pg/mL (P=0.023) (FIGURE 1). A generalized linear model demonstrated a significantly higher predicted response fraction as TPO level declined (P=0.036, Figure 2). TPO level was significantly correlated with lowest effective romiplostim dose (r=0.27, P=0.049).
Optimal TPO Thresholds for Predicting Response: Utilizing Youden's index, the optimally discriminant TPO level threshold for moderate response to romiplostim was ≤457 pg/mL, which predicted a 61% probability of response in those with a TPO level ≤457 pg/mL and a 65% probability of non-response in those with a TPO level >457 pg/mL (Figure 3A). The optimally discriminant TPO level threshold for superior response to romiplostim was ≤260 pg/mL, which predicted a 59% probability of response in those with a TPO level ≤260 pg/mL and a 70% probability of non-response in those with a TPO level >260 pg/mL (Figure 3B). A near trivial response rate was associated with extreme TPO elevations (e.g., 7% for TPO level >1978 pg/mL, Figure 3).
CONCLUSION:
Lower TPO levels predict for a higher likelihood and depth of response to romiplostim in CIT, extreme TPO elevations predict strongly for non-response, and higher doses of romiplostim may be needed in patients with moderate TPO elevations to achieve a response. Considering the significant impact of CIT on patient outcomes as a result of treatment delays and dose reductions, baseline serum TPO level in patients with CIT may be a clinically useful biomarker in predicting response to romiplostim, and therefore potentially useful in guiding patient selection and dosing.
Kuter: Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, CRICO, Daiichi Sankyo, Dova, Genzyme, Immunovant, Incyte, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Principia, Protalex, Protalix, Rigel: Consultancy, Other: grant support and consulting fees; Up-to-Date: Patents & Royalties: Up-To-Date; Rubius: Current equity holder in publicly-traded company; Platelet Disorder Support Association: Membership on an entity's Board of Directors or advisory committees; Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Immunovant, Kezar, Principia, Protalex, Rigel, Takeda (Bioverativ), UCB: Research Funding. Al-Samkari: Novartis: Consultancy; Rigel: Consultancy; Moderna: Consultancy; Argenx: Consultancy; Amgen: Research Funding; Dova/Sobi: Consultancy, Research Funding; Agios: Consultancy, Research Funding.
Romiplostim is a thrombopoietin-receptor agonist (TPO-RA) used off-label for chemotherapy-induced thrombocytopenia. TPO-RAs are well-established in the treatment of numerous etiologies of thrombocytopenia, including immune thrombocytopenia (ITP), aplastic anemia, and thrombocytopenia of chronic liver disease.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal